Sponsored Content by HORIBAReviewed by Olivia FrostJan 14 2026
This article outlines kinetic fluorescence as an analytical method for quantifying non-fluorescent species. The method is applied to the determination of thiamine (vitamin B1) in solution. Fluorescence intensity is tracked as thiamine transforms into fluorescent thiochrome, establishing a relationship between the rate of intensity increase and thiamine concentration. The method demonstrates exceptional sensitivity and selectivity.
Luminescent substances are readily quantified using sensitive fluorescence and phosphorescence methods. Kinetic fluorescent techniques extend the sensitivity advantage of fluorescence analysis to non-fluorescing samples by chemically transforming them into fluorescent species.
For instance, the method can be employed to evaluate pharmaceutical preparations to quantify non-fluorescing thiamine (vitamin B1), which oxidizes to fluorescent thiochrome. Kinetic fluorescence originates from techniques for investigating reaction rates of chemical or physical transformations.
In such studies, the increase or decrease in fluorescence intensity (corresponding to formation or degradation of a fluorescent species) is tracked throughout a reaction to gain insight into the reaction mechanism. Kinetic fluorescence establishes a relationship between the change in fluorescence intensity during a reaction and the concentration of the non-fluorescing reactant. The method’s accuracy and specificity allow quantification of individual components in a multi-component solution without complex separations.
To demonstrate the applicability of kinetic fluorescence for analytical work, this article presents a reaction-rate approach for determining thiamine, an experiment involving commercial multivitamin tablets, and a calibration procedure for thiamine determination using the Fluorolog® modular spectrofluorometer system.
Since thiamine is involved in many metabolic functions, maintaining adequate thiamine dietary levels is vital. Thiamine deficiency may result in degenerative disorders such as edema, muscular atrophy, or beriberi. In addition to thiamine-rich foods (e.g., nuts, vegetables, fish, and liver), thiamine is also incorporated into a wide array of over-the-counter supplements, including vitamin-B complexes and antistress formulations.
Thiamine determination through kinetic fluorescence involves the transformation of thiamine into fluorescent thiochrome, with the changes in fluorescence monitored throughout the transformation. Ryan and Ingle developed the fluorometric reaction-rate technique for thiamine determination, finding Hg2+ to be an effective oxidizing agent, as illustrated in Figure 1.1
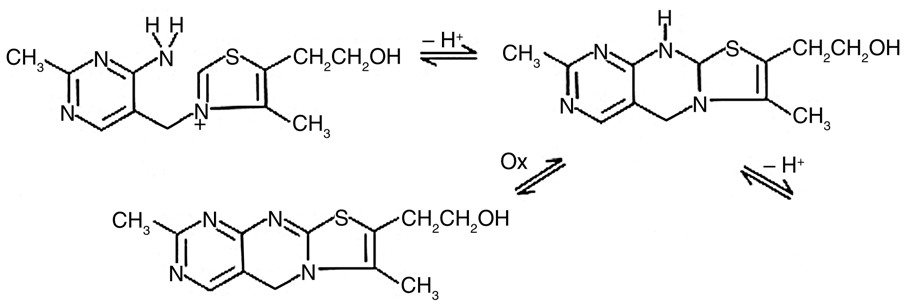
Fig. 1. Reaction of thiamine and Hg2+ to form thiachrome. Image Credit: HORIBA
Ryan and Ingle applied the method to determine thiamine content in a synthetic vitamin-mineral formulation. Bowers adapted their procedure for a student-laboratory experiment to quantify thiamine across various commercial vitamin formulations.2
Experimental method
For the calibration procedure, four thiamine standards were prepared at concentrations of 2.5, 1.25, 0.625, and 0.313 ppm. Mercuric chloride was dissolved in concentrated HCl and diluted to make a 500-ppm Hg2+ solution, and a phosphate buffer (pH = 12.2) was synthesized from Na3PO4·12H2O and Na2HPO4. 1 mL of the Hg2+ solution was added to 1 mL of the thiamine standard in a cuvette, followed by 1 mL of buffer.
The cuvette was placed in the sample compartment of a Fluorolog® spectrofluorometer system. A variable temperature accessory kept the sample temperature at 22.8°C, and a magnetic stirrer ensured continuous stirring during the scan. A single-grating monochromator provided excitation at 365 nm, and a double-grating monochromator tracked fluorescence at 444 nm.
Slits were set to a bandpass of 5 nm. For each calibration standard, the computer acquired a data file representing the fluorescence intensity during the reaction, measured at intervals of 100 ms over a total period of 50 s. The shortest integration time for data acquisition was 1 ms.
Results and discussion
Figure 2 presents four time-based fluorescence scans acquired with the Fluorolog® spectrofluorometer during the oxidation of thiamine to thiochrome. The four scans trace the reaction for standard concentrations of 2.5, 1.25, 0.625, and 0.313 ppm. The reaction rates were calculated from the slope of the intensity-versus-time plots in Figure 2, with their values listed in Table 1.
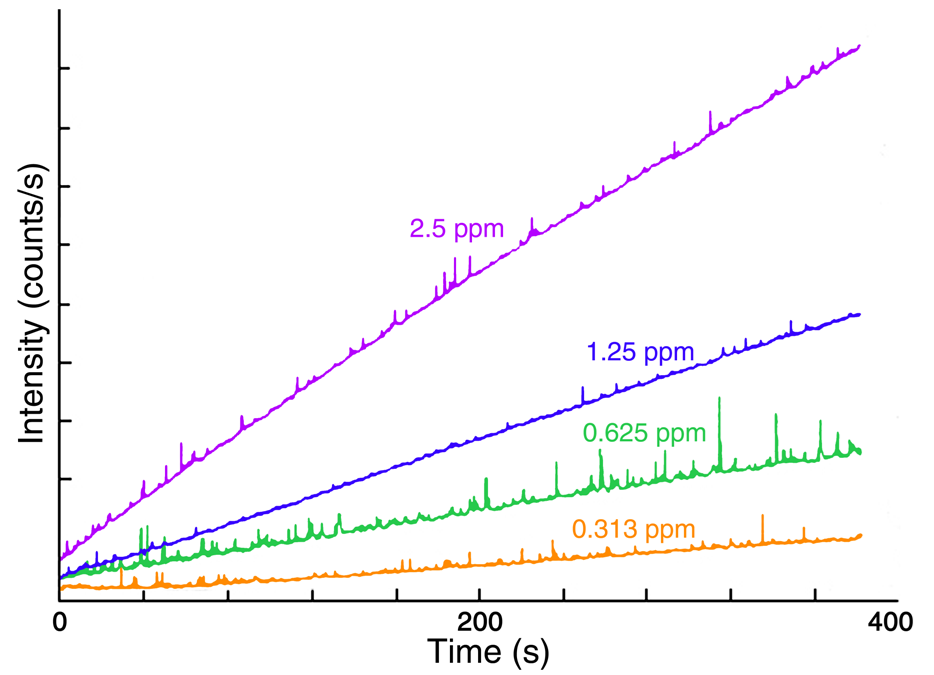
Fig. 2. Plots of fluorescence intensity versus time for the conversion of thiamine to thiachrome for four thiamine standards. Linearity indicates a constant reaction rate for each standard. Image Credit: HORIBA
Table 1. Reaction rates for thiamine standards. Source: HORIBA
| Thiamine concentration (ppm) |
Reaction rate (counts/s2) |
| 2.5 |
4.6 × 103 |
| 1.25 |
2.4 × 103 |
| 0.625 |
1.6 × 103 |
| 0.313 |
5.4 × 102 |
Reaction rates were plotted against concentration, and the best fit was determined via the linear least-squares approach. The resulting calibration curve (Figure 3) can be utilized to quantify trace amounts of thiamine in samples containing additional vitamins and minerals.
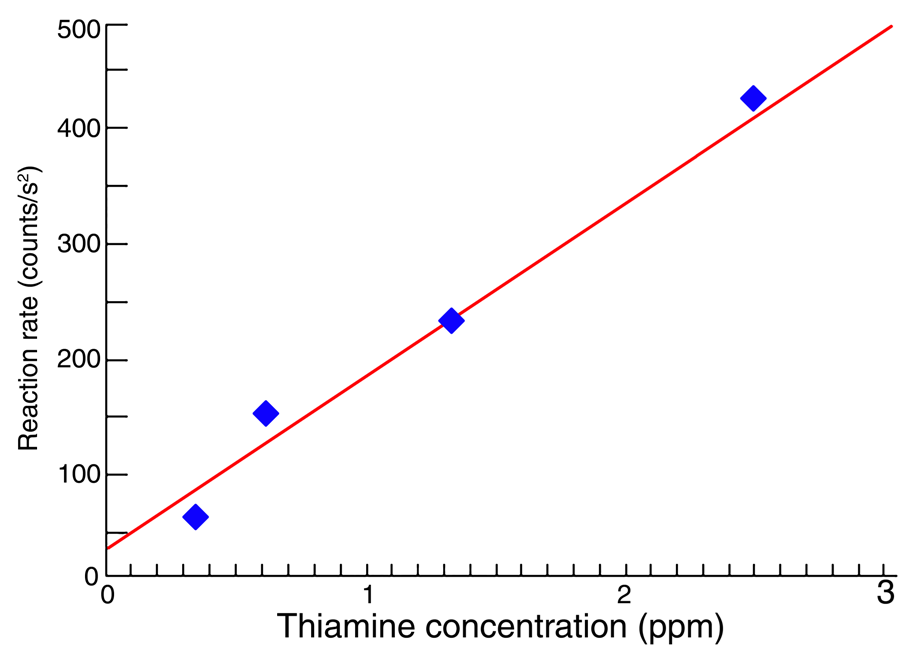
Fig. 3. Calibration curve for reaction-rate determination of thiamine. The line is the best fit to the four thiamine standards. Image Credit: HORIBA
Ryan and Ingle showed that their reaction-rate approach for thiamine determination yielded reasonable results for both commercial and synthetic vitamin-mineral formulations. Kinetic analysis of a synthetic preparation compared favorably with findings acquired using the USP standard analysis method.
Furthermore, the kinetic approach demonstrated advantages in both speed and cost. Bower’s students analyzed five commercial vitamin tablet types using a simplified procedure: samples were run at ambient temperature without stirring, the reaction was monitored on a recorder, and rates were calculated manually from the plots.
Typical findings included determinations within ±2% of the stated amount of thiamine in a “therapeutic” type, within ±5% in a “chewable” type, and within ±6% in a “one-a-day” type. Replication was within ±5% RSD for tablets from the same bottle and from equivalent formulations by different manufacturers.
Ryan and Ingle developed a highly sensitive and selective method for thiamine determination, demonstrating the applicability of kinetic fluorescence in analytical laboratories. Bower’s experiments emphasize the method’s simplicity and reproducibility.
Conclusion
With its sensitive fluorescence measurements, full system control via exclusive FluorEssence™ software, and modular design, the Fluorolog® spectrofluorometer is optimal for kinetic fluorescence determinations. The calibration procedure described in this article can be readily applied to reaction-rate determinations using the Fluorolog® system.
References and further reading:
- Ryan, M.A. and Ingle, J.D. (1980). Fluorometric reaction rate method for the determination of thiamine. Analytical Chemistry, 52(13), pp.2177–2184. https://doi.org/10.1021/ac50063a042.
- Bower, J. J. Chem. Ed. 1982, 59, 975–977.
About HORIBA
Founded in 1953, HORIBA has explored a wide range of unique measurement and analysis technologies to meet global customer needs from 47 group companies and local sites spread across 28 countries and regions. Under the corporate motto Joy and Fun, the company has expanded and refined its core tech-nologies to solve society’s energy issues of today and tomorrow. Our unique measurement and analysis technologies are valued in various fields of society including the three megatrend business fields of Ener-gy & Environment, Biology & Healthcare and Materials & Semiconductor. For more information on HORIBA, visit https://www.horiba.com/int/company/about-horiba/home/
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.