In this article, the variable lipid content present in stratum corneum is visualized and compared at high spatial resolution using atomic force microscopy (AFM). Here, surface features are related to the underlying chemical characteristics using AFM-IR spectra and IR images.
Local lipid concentration corresponding to that of the proteins is evident by monitoring the variation in the band ratio between the methylene C-H (2800–3000 cm-1) and the amide-A (3290 cm-1) stretching bands. Moreover, differentiating localized lipid regions (1732 cm-1) from proteins (1650 cm-1) in corresponding AFM-IR images can now become easier.
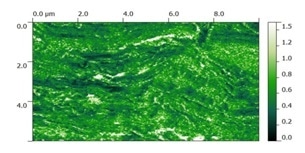
IR chemical contrast image (2930/3290 cm-1) of stratum corneum, showing differences in lipid concentration throughout the sample.
AFM-IR technique
IR spectroscopy is a proven measurement technique in government, academic, and industrial R&D laboratories for polymeric material characterization. Traditional bulk IR spectroscopy has a spatial resolution limited by Abbe diffraction laws to between the ranges of 3-10 µm, based on the technique employed. AFM is a proven nanoscale imaging technique capable of providing a topographic map of the surface of a sample with a high spatial resolution.
Until recently, chemical characterization of the material beneath the tip was not within the scope of AFM – a major drawback of this technique. When an IR source is used along with AFM, the resulting AFM-IR technique overcomes the diffraction limit of traditional IR spectroscopy by several orders of magnitude without compromising the high-resolution imaging capabilities of the AFM technique.1
The following are the key capabilities of nanoIR spectroscopy:
- The chemical composition of biological matters such as cells and tissues can be uniquely and clearly identified using nanoIR spectroscopy.
- nanoscale IR spectroscopy allows easy material identification by directly correlating to bulk FTIR libraries.
- Tapping AFM-IR provides <10 nm chemical mapping spatial resolution with simultaneous mechanical property mapping.
Nanoscale chemical characterization of stratum corneum
The stratum corneum (SC) is the outermost skin layer that acts as a natural barrier to prevent the entry of foreign substances and keeps the homeostasis of the body – which is vital in maintaining the health of an individual.
SC contains a brick-and-mortar structure of lipids and corneocytes that is produced from the skin layers underneath [1]. This is partly due to the natural shedding and reproduction of the epidermis. SC is regarded as the more rigid skin layer than the rest of the skin structure. Its moisture and lipid content fluctuates in response to the external environment.
While chemical characterization of SC has been the subject of intense research, observing structures tinier than the size range of 3–10 µm is an impossible task with existing IR microspectroscopy due to its diffraction-limited resolution.
Here, using AFM-IR spectroscopy enables examining the distribution of the lipid content in a SC specimen at a spatial resolution significantly higher than that can be achieved by traditional Fourier transform infrared microscopy.
A Kapton tape strip was used to obtain SC from a subject’s skin surface. The collected SC specimen was then directly placed onto a ZnSe prism. The AFM technique was then used to image the clusters of SC cells remained attached to the surface of the prism after the removal of the tape (Figure 1A).
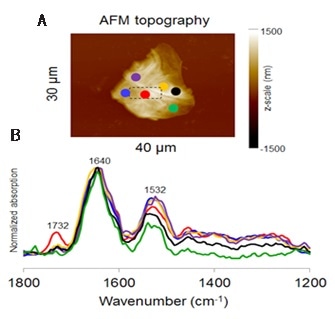
Figure 1. Point-and-click spectral acquisition over a large area of a SC specimen on a ZnSe prism; the area in the dashed box is enlarged and displayed in Figure 2. (Low wavenumber spectra are normalized to 1640 cm-1)
References
- Bonte, et. al. J. Cosmet. Derma. 2007, 6, 75
- Mendelsohn, et. al. PCCP. 2000, 2, 4651
About Bruker Nano Surfaces and Metrology

Bruker’s suite of fluorescence microscopy systems provides a full range of solutions for life science researchers. Their multiphoton imaging systems provide the imaging depth, speed and resolution required for intravital imaging applications, and their confocal systems enable cell biologists to study function and structure using live-cell imaging at speeds and durations previously not possible. Bruker’s super-resolution microscopes are setting new standards with quantitative single molecule localization that allows for the direct investigation of the molecular positions and distribution of proteins within the cellular environment. And their Luxendo light-sheet microscopes, are revolutionizing long-term studies in developmental biology and investigation of dynamic processes in cell culture and small animal models.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.