This article is based on a poster originally authored by Sicong Zhang, Xuzhen Zhou, Chanyu Wang, Danxi Li and Mandy Xu.
Introduction
With their ability to target typically "undruggable" targets, oligonucleotide therapies (ONTs) have become a promising new approach to drug development.
After entering cells, ONTs are carried through endosomes and destroyed in the lysosome.
Conjugated ONTs, such as GalNAc-siRNA and antibody-oligonucleotide conjugates, also require the lysosome to release the oligonucleotide into the cytosol for proper activity.
As a result, examining the stability of ONTs in the lysosome is crucial for understanding their transport, degradation, and immune toxicity triggering, providing recommendations for enhanced therapeutic efficiency and safety in drug design.
This article presents an LC-MS-based experiment system for the in vitro lysosome stability testing of ONTs.
Methods
- The researchers evaluated the stability of commercially available siRNAs (patisiran, inclisiran, and tivanisiran) in the human lysosome.
- To focus on nuclease cleavage, the specificity of nuclease degradation in the lysosome was investigated by adding nuclease inhibitors to the system. The ribonucleoside vanadyl complex (RVC), a non-protein inhibitor, was selected to limit nuclease activity specifically in the lysosome. The inhibitory capacity of two protein-based inhibitors was also evaluated for comparison.
The nuclease resistance of ONTs was analyzed in an in vitro lysosome system
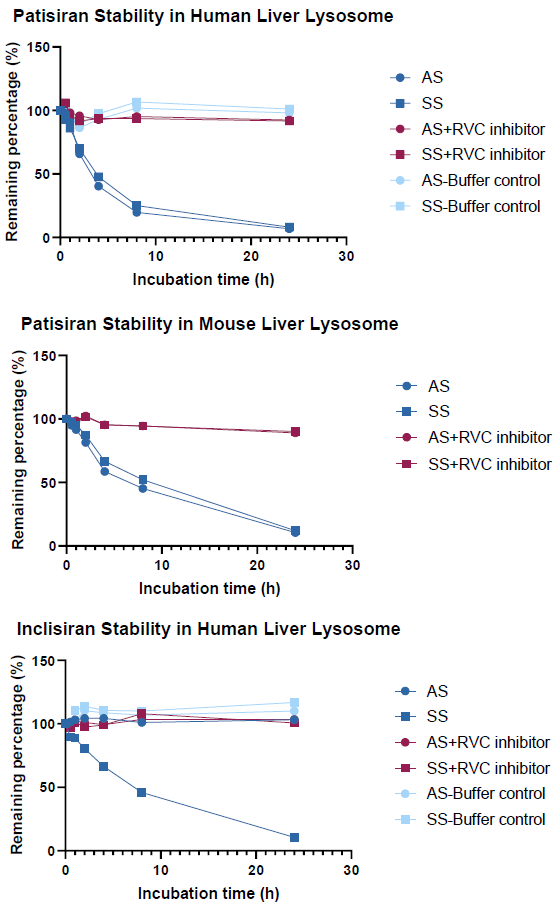
Image Credit: Pharmaron
Assessing ONT stability in an in vitro lysosome system:
The siRNA medicines patisiran and inclisiran were degraded by lysosomes after 24 hours to 6.76-11.92 % of their original concentration, with T1/2 ranging from 3.25-7.78. Given lysosomes' high protein degradation capacity, protein-based inhibitors may not be effective in inhibiting lysosomal enzymes.
As a result, RVC, a non-protein inhibitor, was chosen to specifically inhibit nuclease activity in the lysosome. The RVC inhibitor effectively reduced nuclease activity in the system.
AS: antisense strand; SS: sense strand.
The RVC inhibitor effectively inhibited nuclease activity in lysosome while protein-based inhibitors did not.
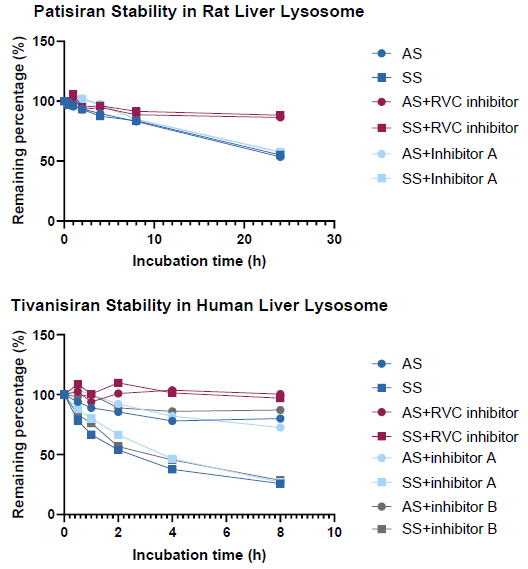
Image Credit: Pharmaron
Assessing the inhibition capacity of nuclease inhibitors in in vitro lysosome system:
The RVC inhibitor efficiently inhibited nuclease activity in an in vitro lysosome system. The two protein-based inhibitors did not reduce nuclease activity.
AS (antisense strand), SS (sense strand), and inhibitor A: Ribonuclease inhibitor (RNasin®); B: RNase inhibitor (SUPERase∙InTM).
Adding the RVC inhibitor to the lysosome system slightly impacted the degradation of non-nucleic acid molecules
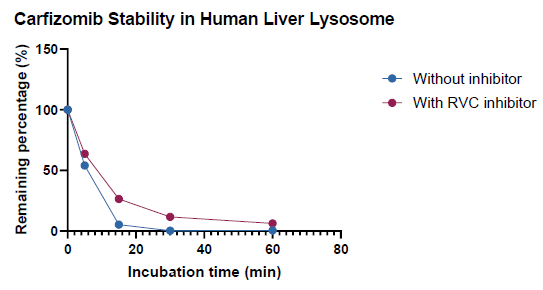
Image Credit: Pharmaron
Assessing the inhibition capacity on non-nucleic acid compound:
The RVC inhibitor somewhat reduced the breakdown of carfizomib, a tetrapeptide, in the system.
The RVC inhibitor must be pre-warmed to 65 °C for disintegration. The high temperature of the inhibitor solution, even in a tiny volume, may reduce overall enzyme activities.
Conclusion
- An in vitro experiment system was established to investigate ONT stability in the lysosome.
- The RVC inhibitor was chosen for studying ONT nuclease resistance in lysosomes because of its inhibitory potency and selectivity.
- Protein-based nuclease inhibitors were unsuitable for the lysosome system.
- This assay technique is likely to serve as a screening platform for novel ONTs, hence facilitating medication development.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development and manufacturing service capabilities throughout the entire drug discovery, preclinical and clinical development process across multiple therapeutic modalities, including small molecules, biologics and CGT products. With over 17,000 employees, and operations in China, the U.S., and the U.K., Pharmaron has an excellent track record in the delivery of R&D solutions to its partners in North America, Europe, Japan and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Jan 9, 2026