This article and associated images are based on a poster originally authored by Kieran Casey, Yuzhou Xu, Jichuan Zhang, Shuyue Wang, Yanfei Hu, Ye Tian, Min Lyu, Ziyu Chen, Ya Xu, Qikuan Chen and Yinfei Yin and presented at ELRIG Drug Discovery 2025 in affiliation with ChemPartner European Strategic Hub and ChemPartner Co., Ltd.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Targeted protein degradation (TPD) is an emerging strategy designed to develop novel therapies for cancer and other diseases. In recent years there has been significant interest in this field of drug discovery, and at the forefront of TPDs are proteolysis-targeting chimeras (PROTACs) and molecular glue degraders (MDGs) (Fig. 1). PROTACs are a novel class of heterobifunctional molecules that bind to both the E3 ligase and the target protein and contain three components; the protein of interest (POI) binding moiety, a linker, and E3 ubiquitin ligase binding moiety. Once bound, a ternary complex is formed (POI-PROTAC-E3 ligase), which hijacks the ubiquitin-proteasome system and is essential in driving proteasomal degradation of the target protein. Unlike conventional inhibitors that disable target proteins by direct binding, PROTACs are event-driven, meaning that a single PROTAC molecule can induce the degradation of multiple target protein molecules. This catalytic mechanism allows PROTACs to achieve complete inhibition of target proteins at lower concentrations compared to conventional inhibitors, making them a promising therapeutic approach for previously considered "undruggable" targets.
Despite the promising results observed with PROTACs, several challenges remain; the large molecular weight of PROTACS can lead to poor cell permeability and bioavailability, and the current lack of high-throughput, quantitative assays for evaluating PROTAC efficacy hinders structure-activity relationship (SAR) studies.
Using cost-efficient, high-throughout assays, ChemPartner has developed a comprehensive suite of preclinical models for PROTAC evaluation, encompassing both in vitro and in vivo approaches. Using our range of validated assays, we can provide valuable insights into PROTAC efficacy, facilitating SAR studies and elucidating the relationship between molecular structure and activity. Furthermore, our extensive in vivo capabilities facilitates the investigation of a PROTACs PK/PD profile, toxicity and efficacy, providing a powerful tool accelerate PROTAC development whilst reducing the fail rate of PROTACs in clinical trials.
This poster will focus on a number of key assays that ChemPartner has validated from its PROTAC evaluation platform, which encompasses what we have defined as the “11 Key Questions for PROTACs”. Each question addresses a key aspect of a PROTAC’s biological activity or characteristics, thereby narrowing down the challenges in PROTAC SAR research.
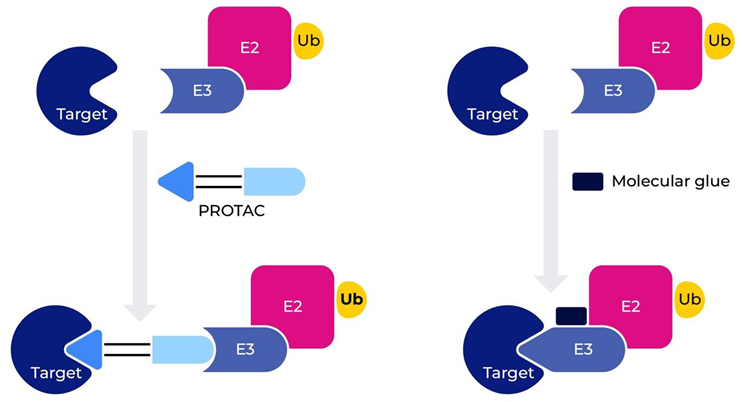
Fig. 1. Schematic representation of PROTAC and Molecular Glue. Image Credit: https://doi.org/10.1038/s41392-022-00966-4
The “missing bridge” in PROTAC SAR
For PROTAC, the SAR is much more difficult than traditional small molecules (inhibitors), since the connection between biochemical and cellular data is not clear. Currently, Western blot is used to evaluate the effectiveness of PROTAC degradation of a target protein, but there is a wide gap between target protein binding and target protein degradation; the “missing bridge”. The high complexity of a PROTAC molecule compared to a traditional small molecule means that robust assays that bridge the gap between the biochemical and cellular data need to be introduced.
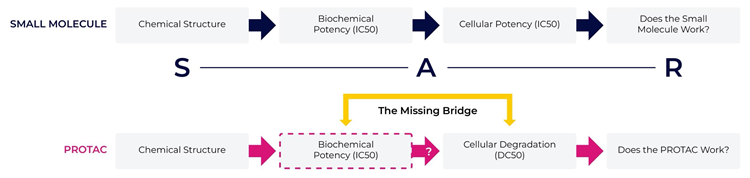
Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
11 key questions – building the “missing bridge”
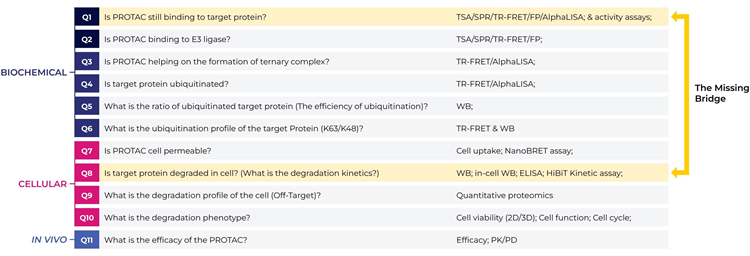
Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Results
Biochemical assays
Q2 – Is PROTAC binding to E3 ligase?
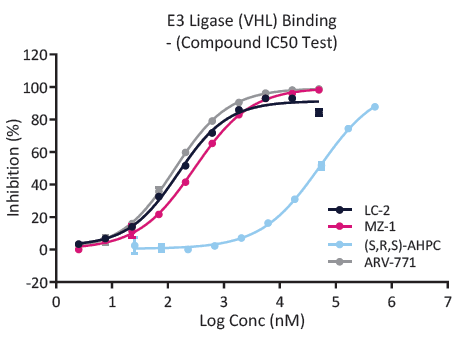
Fig. 2. An example of a well-established, ready-to-go E3 ligase assay at ChemPartner. ChemPartner generates highly active E3 ligases, for both multi-subunit E3 complexes, and single protein E3, and are exploring more E3 ligases. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Q3 – Is PROTAC helping the formation of the ternary complex?
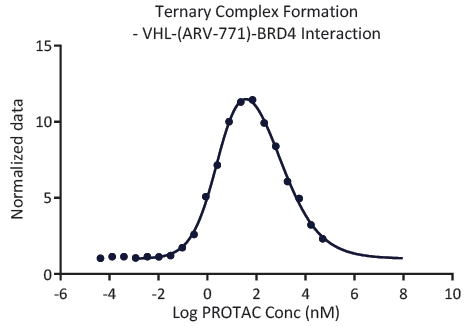
Fig. 3. A typical "bell-shaped dose response curve" formed by a PROTAC in a ternary complex formation assay. At ChemPartner we utilize techniques such as TR-FRET and/or AlphaLISA to demonstrate ternary complex formation. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Q4 – Is the target protein ubiquitinated?
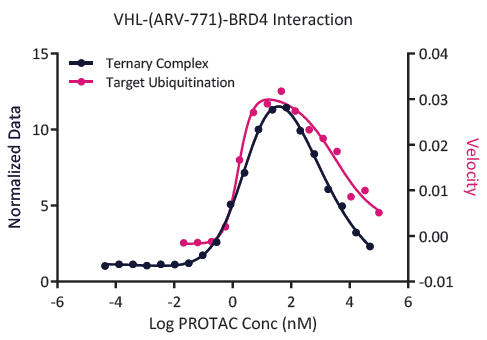
Fig. 4. A typical "bell-shaped-curve" formed by a PROTAC in a ternary complex formation assay. vs. ubiquitination assay. This is an enzymatic assay, and for a "good" PROTAC, the ubiquitination peak is similar to the ternary complex peak. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Q5 – What is the ratio of ubiquitinated target protein?
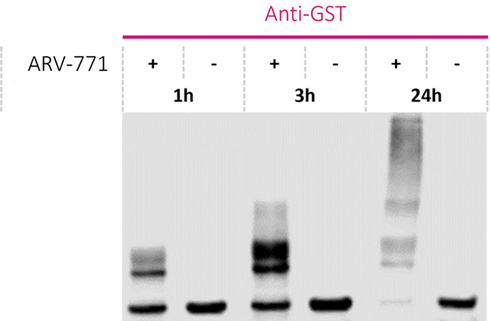
Fig. 5. "Ladder pattern" of target protein formed by a PROTAC in a ubiquitination assay. Protein ubiquitination increases over time, and for some PROTACs, complete poly-ubiquitination of the target protein can be observed. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Cellular assays
Q7 – Is PROTAC cell permeable?
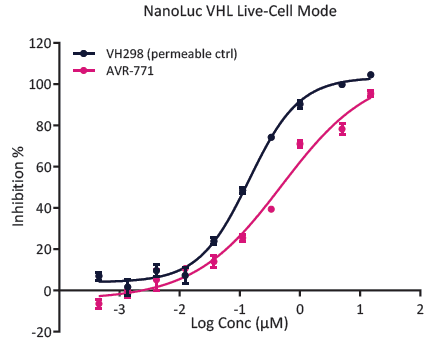
Fig. 6. NanoBRET assay for PROTAC evaluation. Intracellular availability can be measured using live-cell/permeabilized-cell modes, providing a measure of PROTAC permeability. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Q8 – Is the target protein degraded?
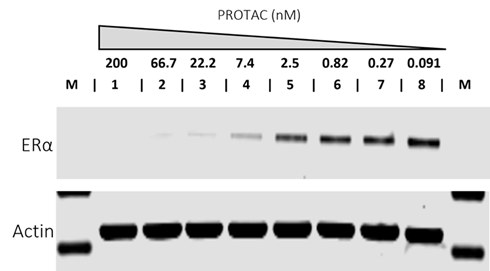
Fig. 7. Western blot assay on a cell line treated with PROTAC. Target protein degradation increases in line with the concentration of PROTAC used in the assay. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
In vivo assays / proteomic analysis
Q11 – What is the efficacy / PK profile of the PROTAC?
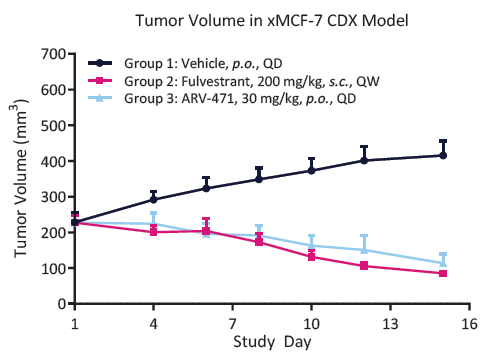
Fig. 8. XMCF-7 tumor growth kinetics. Treatment with Fulvestrant and ARV-471 significantly reduced the tumor volume in immunodeficient mice (p<0.0001). Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
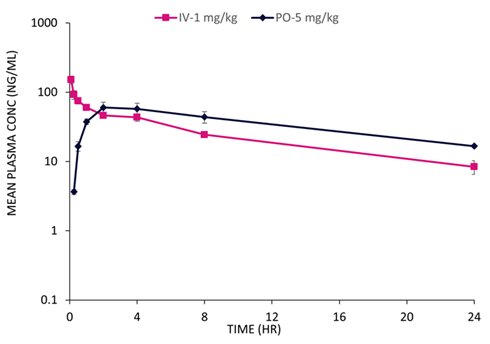
Fig. 9. PK plasma profile following intravenous and oral administration of a PROTAC. Typical intravenous and oral plasma profiles were observed in dogs. Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
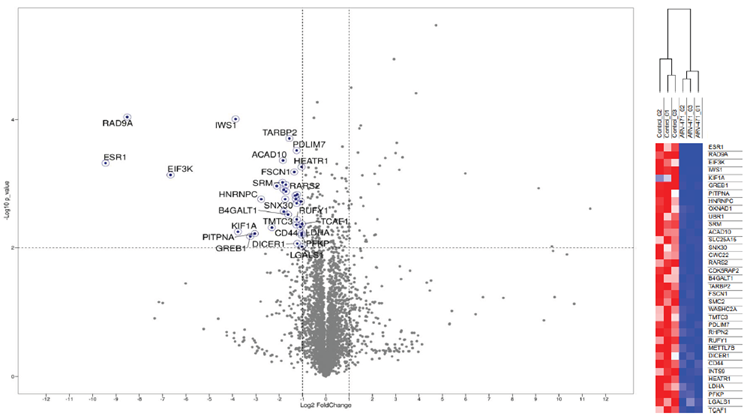
Fig. 10 (left) and 11 (right). Proteomic analysis of ARV-471 treated XMCF-7 tumors. Volcano plot (left) of significantly deregulated proteins identified in the control vs ARV-471 tumor lysates. Hierarchical clustering (with Euclidean distance) (right) of the top 35 down-regulated proteins in control vs. ARV-471 (>1 unique peptide, P-value <0.001, ≥2-fold decrease in protein level). Image Credit: Image courtesy of Kieran Casey et al., in partnership with ELRIG (UK) Ltd.
Summary
We have developed a comprehensive assay platform to characterize various aspects of PROTACs and validate their role in target protein degradation (TPD). Data presented demonstrates that using our assay platform we can validate the following key processes in PROTAC evaluation; PROTAC binding to E3 ligase, PROTAC aiding in the ternary complex formation, and target protein ubiquitination and degradation in the cell. In addition, this platform can also be applied to evaluate molecular glue degraders; another modality that utilizes a TPD mechanism. By providing a wealth of information, particularly via high-throughput in vitro assays, this powerful platform significantly accelerates the development of both PROTACs and molecular glue degraders. We have developed and validated a plethora of robust, in vivo models that can complement our in vitro assay platform. Using one of our subcutaneous CDX models, we have confirmed the PK profile of PROTACs in vivo, as well as defining their anti-tumor efficacy. Furthermore, tumors taken from control and ARV-471 treated mice, were subjected to proteomic profiling. Quantitative proteomics revealed that the most significantly downregulated protein was, unsurprisingly, ESR1 (estrogen receptor α) (-9.45 log fold change), with GREB1 (a known target of ER) also significantly downregulated (-3.24 log fold change). Hierarchal clustering also clearly grouped the tumor samples based on treatment, which again validates the robust and reproducible nature of the in vivo models and downstream analysis applied. The quantitative data generated from these assays plays a crucial role in optimizing these TPD drugs. This data is also valuable for building in silico models and training AI algorithms for future drug discovery efforts. Beyond PROTAC: The limitations of PROTAC are that they can lead to the identification of compounds that are outside of Lipinski’s rule of five, have molecular weights >500 Da and in most cases, are not orally bioavailable. An alternative to PROTACs are molecular glue degraders; MGDs generally have molecular weights of <500 Da, satisfy Lipinski’s rule of five and are orally bioavailable. ChemPartner have developed and validated a screening tree for MGDs, allowing a plethora of proteins that do not have a well-defined binding pocket (currently considered undruggable) to be exploited.
References
- Zhao, L., et al. (2022). Targeted protein degradation: mechanisms, strategies and application. Signal Transduction and Targeted Therapy, 7(1). DOI: 10.1038/s41392-022-00966-4. https://www.nature.com/articles/s41392-022-00966-4.
- Morpheus. Available at: https://software.broadinstitute.org/morpheus11.
About ChemPartner
Over the past two decades, ChemPartner has evolved from a chemistry-focused service provider into a research-driven innovation partner, offering an integrated portfolio that spans drug discovery, development, and manufacturing across both small-molecule and large-molecule therapeutics.
Shanghai ChemPartner, encompassing ChemPartner and ChemPartner Biologics, operates as a global CRO and CDMO delivering comprehensive support throughout the drug development lifecycle. The organization provides science-led, technology-enabled services including discovery chemistry, biology, pharmacology, DMPK, and exploratory toxicology, alongside biologics discovery, CMC development, and manufacturing capabilities.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Jan 14, 2026