This article and associated images are based on a poster originally authored by Wambeke E, Boribong BP, Sinha H, Patel M, Vo PQN, Chin AB, Ellouzi A, Barlan K and Hirukawa A and presented at ELRIG Drug Discovery 2025 in affiliation with Abbvie and DropGenie.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Arrayed gene editing screens enable high dimentional data generation
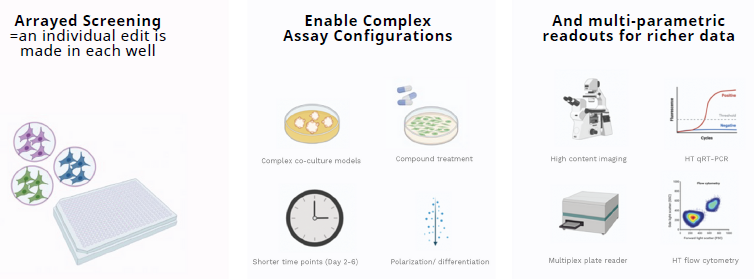
Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
Editing biologically relevant models like primary cells at scale is challenging
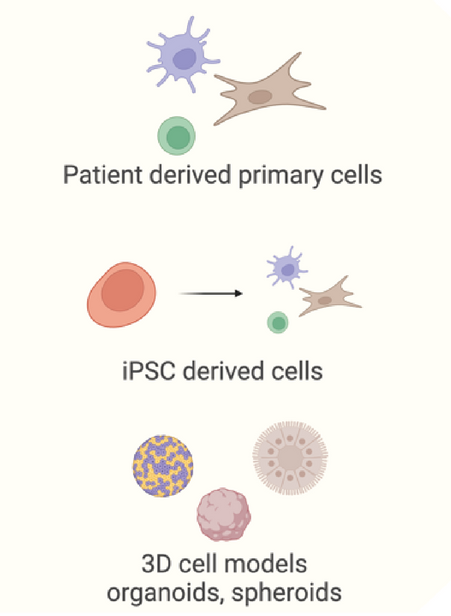
Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
Sample scarcity
- Limited proliferative runway
- Bottlenecks to donor access
Cost
- Expensive to culture and procure cells
- Reagent costs rise quickly with scale
Digital microfluidics enables 100x miniaturized cell engineering
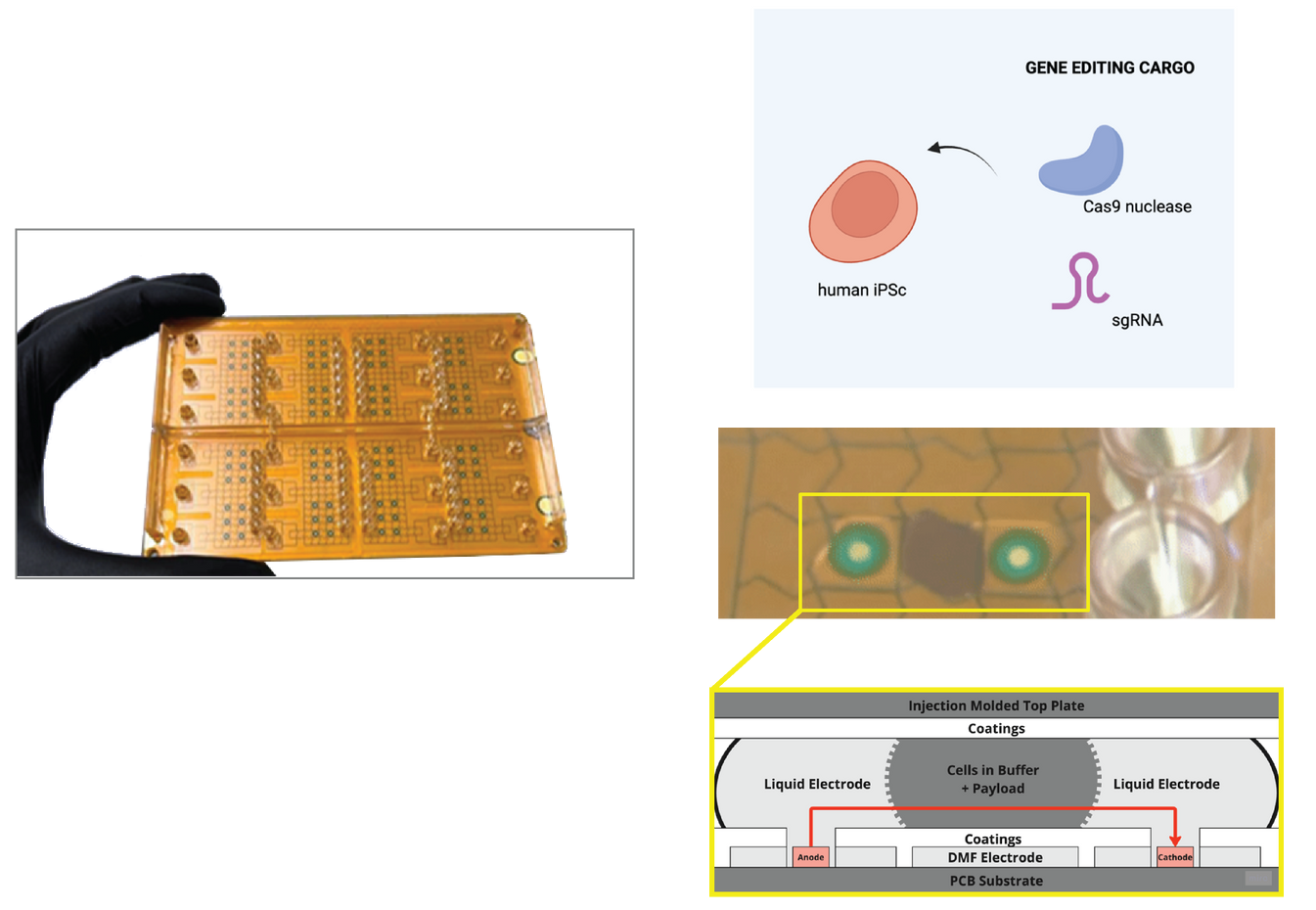
Digital microfluidics enables highly miniaturized electroporation for cell engineering. DropGenie digital microfluidic (DMF) cartridges (left) use patterned electrodes and a modular top plate to manipulate nanoliter-scale droplets. Editing cargo (Cas9 nuclease and sgRNA) and human cells are combined in discrete droplets (top right) and electroporated within microchambers (center). Cross-sectional schematic (bottom) illustrates the DMF architecture, with cells and reagents confined between the coated top plate and PCB substrate. This platform enables 100× miniaturization compared to conventional systems, reducing input requirements to as few as 10,000 cells per edit. Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
With seamless integration into automation workflows for scale
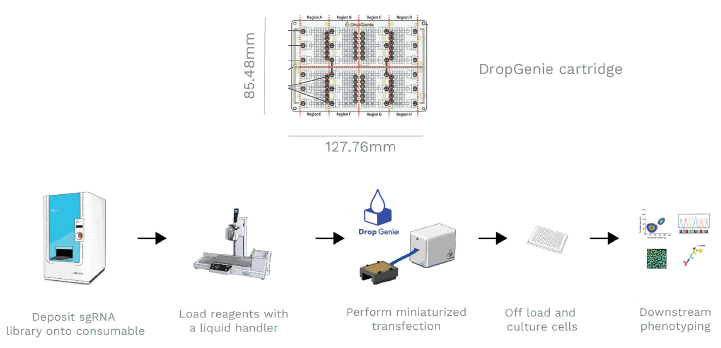
DropGenie Workflow. Guides are deposited onto the DropGenie Chip using an ECHO acoustic dispenser (BCLS), and cells with payload are loaded into the cartridge with a Liquid Handler (Integra Assist+). The system runs with user-defined parameters, after which cells are plated for culture and recovery. Transfection efficiency is then assessed via flow cytometry, sequencing, or fluorescence microscopy. Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
For robust, miniaturized editing of primary immune cells
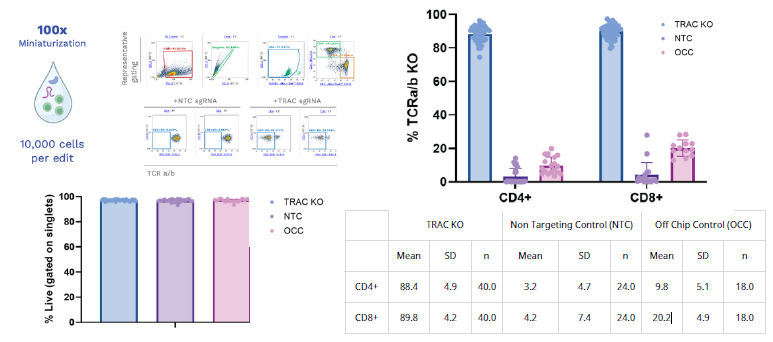
Miniaturized DMF electroporation enables efficient TRAC knockout in primary human T cells. Representative flow cytometry gating strategy (top left) shows singlet, live, and CD4⁺/CD8⁺ T cell populations used to quantify TCRα/β knockout efficiency. Example plots demonstrate loss of TCR expression following TRAC sgRNA editing compared to non-targeting control (NTC). Editing efficiency (top right) was robust in both CD4⁺ and CD8⁺ subsets, reaching ~88–90 % KO across 40 replicates, while controls exhibited only background signal. Cell viability (bottom left) remained >90 % across all conditions. Summary statistics (bottom right) confirm high knockout efficiency with low variability (CD4⁺ mean 88.4 % ± 4.9 %; CD8⁺ mean 89.8 % ± 4.2 %) and strong reproducibility across donors. A total of 10,000 cells per edit were used, representing a 100× reduction in input compared to conventional electroporation systems. Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
Study objectives and methods
- Reduce gene editing cell input requirements to enable use of patient-derived material at scale
- Quantify the cost saving of efficient editing through reagent and workflow miniaturization
- Optimize a miniaturized, automation-compatible CRISPR editing protocol for primary cell models relevant to Rheumatoid Arthritis (RA)
- Primary Fibroblast Like Synoviocytes (FLS) cells from RA donor, used at 70 % confluence
- Validate platform with mRNA transfection
- GFPmRNA (Tri-Link). Evaluate %GFP Imaging: Operetta CLS (Revvity), 24 hours post transfection
- Achieve robust and reproducible gene knockout
- Multi-guide CD81 gene knockout
- synthetic guide RNA (IDT), Cas9 nuclease (IDT), Electroporation enhancer (IDT)
- 3 sequences targeting CD81 (IDT)
- At 7 d, cells fixed and stained with Hoechst (nuclei) and anti-CD81 + fluorescent secondary. Imaging: Operetta CLS (Revvity), 5× magnification, 2 fields/sample. %CD81⁻ quantified as (#CD81⁺/# nuclei).
Miniaturized CRISPR editing in primary synoviocytes
High-efficiency delivery of GFP mRNA into primary fibroblast-like synoviocytes
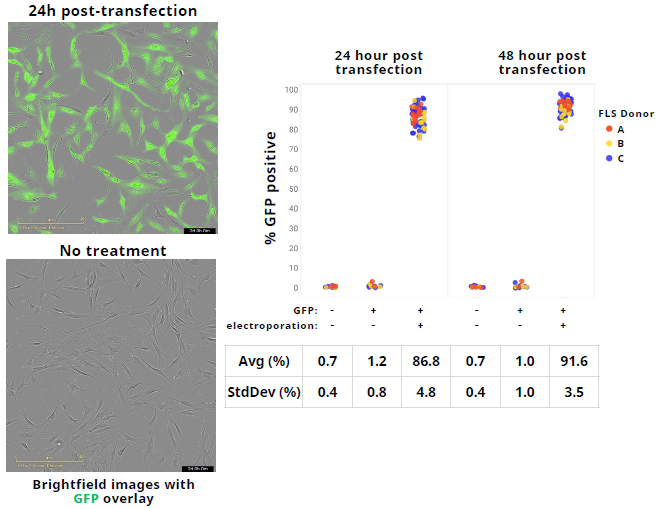
Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
High-efficiency CRISPR knockout of CD81 in primary fibroblast-like synoviocytes
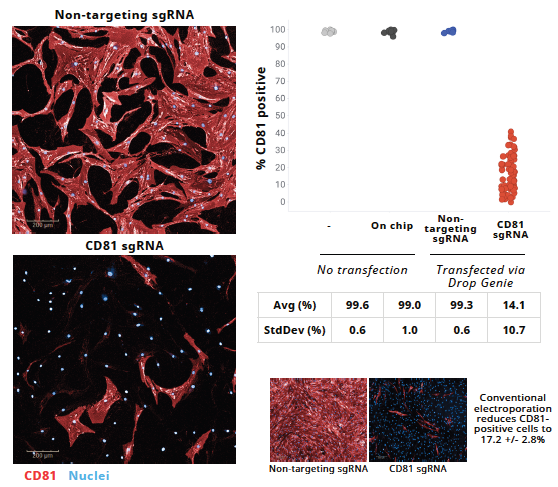
Image Credit: Image courtesy of Wambeke E et al., in partnership with ELRIG (UK) Ltd.
Quantification of cost savings per RNP mediated CRISPR KO edit

Conventional Electroporation
Cell input/ reaction
Source: ELRIG (UK) Ltd.
| . |
. |
. |
| Primary FLS cells |
25,000 |
3,000 |
Reagent cost/ reaction
Source: ELRIG (UK) Ltd.
| . |
. |
. |
| Cas9 nuclease |
30 pmol |
3 pmol |
| sgRNA |
60 pmol |
5 pmol |
| Electroporation Enhancer |
30 pmol |
3 pmol |
| Total cost per reaction |
$17.87 |
$1.69 |
Conclusions
- Miniaturized DMF electroporation enables high efficiency CRISPR KO in primary FLS
- >8× reduction in cells per reaction (3,000 FLS cells / edit)
- GFP mRNA delivery: >90 % transfection
- CD81 KO up to 85 % across Rheumatoid Arthritis donors
- Automation-integrated workflow ensures precision, reproducibility and scale
- Consumable compatibility wih ECHO 655T
- Seamless integration with Integra Assist+ Liquid handler minimizes handling loss
- >10× cost savings while preserving scarce patient cells
- Cost reduction from $17.87 → $1.69 per edit
- Unlocks scalable functional genomics in Rheumatoid Arthirtis-relevant primary cells, enabling discovery of new therapeutic targets
Disclosures
AbbVie authors may hold stock and shares in AbbVie. DropGenie authors may hold shares in DropGenie. Platform used for Research Use Only (RUO).
About DropGenie
DropGenie has optimized the miniaturization and parallelization of cell engineering reactions using patented chip technology. This is achieved by precisely delivering a wide range of editing reagents into diverse cell types at low volumes, enabled through intelligent tuning of electrical parameters and a proprietary electrode configuration.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Jan 6, 2026